Strontium Forms a Cation or Anion
The general electronic configuration of s-block elements is noble gasns1 for alkali metals and noble gas ns2 for alkaline earth metals. Na S 2-Na 2 S Mg 2 O 2-MgO Fe 3 O 2-Fe 2 O 3.

Ions And Ionic Compounds Ions Atoms Or Groups Of Atoms That Have A Positive Or Negative Charge Forms When An Atom Or Group Of Atoms Loses Or Gains Electrons Ppt Download
There are separate procedures for detecting cations and anions called the Cation Analysis and Anion Analysis.
. It is a. It usually appears as a pale greenish yellow dilute solution. Methods for the analyses of solu ble sorbed and total concentrations of 34 elements are also included.
It also forms a chloride in the form XCl2 and an oxide in the form XO. Binary Ionic Compounds Containing a Metal and a Nonmetal. Calculate Zeff for the 4s electron in a copper atom Cu.
The phosphide anion. So here are 4 experiments that we can do to improve our learning in chemistry. Now that the cation and the anion are identified obtain the chemical formula of the salt by balancing the charges of the cation and anion.
Salinity carbonate and gypsum soil pH and acidity lime requirement cation and anion exchange capacities and organic matter. Hier sollte eine Beschreibung angezeigt werden diese Seite lässt dies jedoch nicht zu. Calculate Zeff for a valence electron in an oxygen atom.
UNIT 10 After studying this unit you will be able to describe the. It is also known as liquid bleach. Na SO 4 2-Na 2 SO 4.
A thorough presentation of analytical methods for characterizing soil chemical properties and processes Methods Part 3 includes chapters on Fourier transform infrared Raman electron spin resonance x-ray photoelectron and x-ray absorption fine structure spectroscopies and more. It has a sweetish and chlorine-like odour. Hydrogen forms the only charge-1 cation that has no electrons but even cations that unlike hydrogen retain one or more electrons are still smaller than the neutral atoms or molecules from which they are derived.
Express your answer numerically. Let us discuss about the Qualitative Analysis of Cations. Since the electric charge on a proton is equal in magnitude to the charge on an electron the net electric charge on an ion is equal to the number of protons in the ion.
CationA positively charged ion as opposed to an anion. The total positive charge must balance the total negative charge. Qualitative Analysis of Cations Preliminary Tests.
Yang Shao-Horn studies materials for electrochemical and photoelectrochemical energy storage and conversion which is centered on examining the influence of surface chemistry and electronic structures of thin films and nanomaterials on lithium storage and catalytic activity of small molecules of energy consequence and applying. Spectator ionAn ion that exists as a reactant and a product in a chemical reaction. Express your answer using a chemical symbol.
A proper ionic formula has a cation and an anion in it. The element is a liquid at room temperature. The physical examination of the unknown.
It consists of hypochlorite anion and sodium cation. Additionally these chapters include useful background information on the chem istry of the elements. UNIT 10 After studying this unit you will be able to describe.
You can also identify the cation first and then move on to identifying the anion. Strontium and barium have much lower abundances. An ionic compound is never formed between two cations only or two anions only.
AnionA negatively charged ion as opposed to a cation. List of Chemistry Experiments for High School - Fun and Simple Learning through experiencing is such an effective way of absorbs new information and turn it into a long-term memory. Beryllium is rare and radium is the rarest of all comprising only 1010 per cent of igneous rocks Table 102 page 299.
Chemical formulas for ionic compounds are called ionic formulas. The key to writing proper ionic formulas is simple. NH 4 SO 4 2-NH 4 2 SO 4 Nomenclature of Ionic and Covalent Compounds.
For example if your cation is Fe 3 and your anion is Cl the chemical formula of the salt will be FeCl 3. The -ate forms formula and charge must be memorized. Na Cl-NaCl Ca 2 Br-CaBr 2.
What is the identity of this element. Mg 2 NO 3-MgNO 3 2. PrecipitateTo come out of a liquid solution into solid form.
Strontium and barium have much lower abundances. It is widely used as a cleaning agent or disinfectant and bleaching agent. Beryllium is rare and radium is the rarest of all comprising only 1010 per cent of igneous rocks Table 102 page 299.
InsolubleThat which cannot be dissolved. The general electronic configuration of s-block elements is noble gasns1 for alkali metals and noble gas ns2 for alkaline earth metals. PrecipitateA solid that exits the liquid phase of a solution.
It is an anhydrous unstable compound which can decompose explosively. Some preliminary tests needs to be done before doing the analysis of cations.
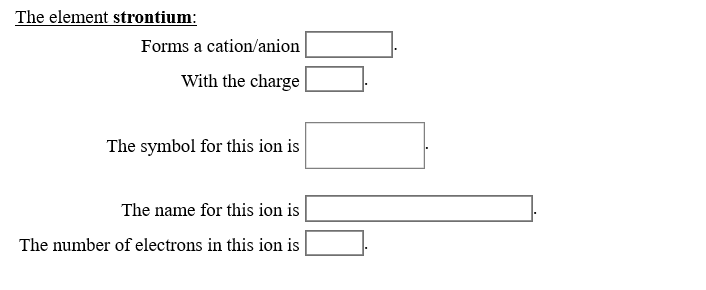
Solved The Element Strontium Forms A Cation Anion With The Chegg Com
Strontium Ii Cation Sr Chemspider
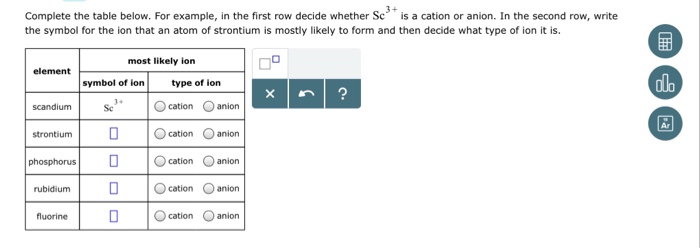
Solved Complete The Table Below For Example In The First Chegg Com

No comments for "Strontium Forms a Cation or Anion"
Post a Comment